List of data collected through patient-reported surveys as well as clinical data.
Our data are collected primarily from patients and include patient-reported outcome measures (PROM) of pain and function, early post-operative adverse events, and implant failures.
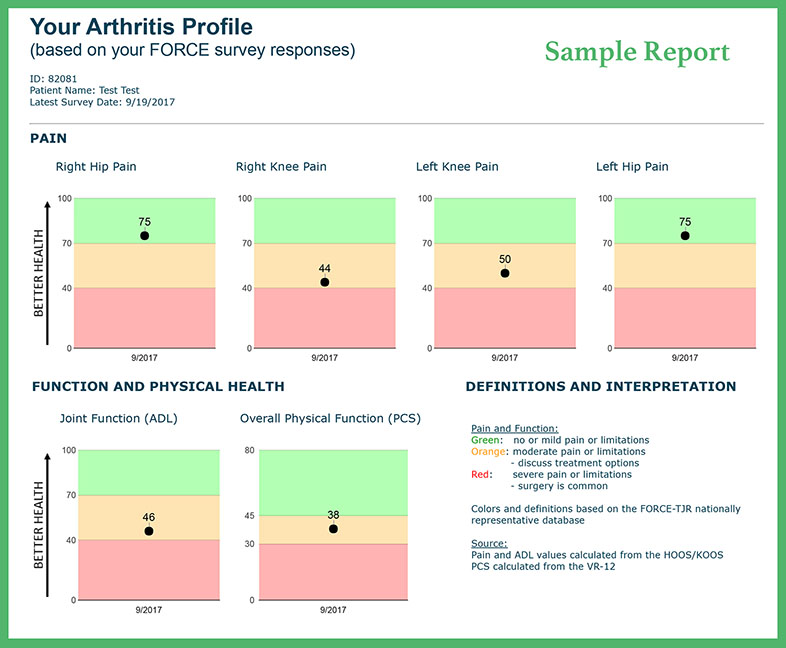
In collaboration with patient and surgeon advisors, FORCE-TJR Research designed a real-time patient-reported pain and function monitoring report and tested it in surgeon offices. Scored and trended data, compared to national benchmarks, are available at the clinical visit to support treatment discussions between patients and surgeons.
In a work funded by the Patient-Centered Outcomes Research Institute (PCORI), we are expanding web-based, direct-to-patient informatics to provide individualized patient care plans (the Osteoarthritis Careplan or A.S.K.) to support treatment discussion between patients and clinicians. These new reports (as shown on the left) are being developed in partnership with our patient and family advisors.
Finally, a patient-centered phone app has been designed to capture daily patient knee and hip arthritis symptoms, as well as standardized PROMs.
|
Data collected |
|
PROMs: HOOS/KOOS or HOOS/KOOS – JR |
|
General health related quality of life |
|
Medical and Musculoskeletal risk factors |
|
Adverse Events |