FORCE-TJR merges patient-generated data with administrative and clinical data to serve clinical and policy evaluations of devices, surgical practice, and timing and use of orthopedic interventions.
Comprehensive PROM collection across a range of orthopedics specialties |
|
| Orthopedic specialty | Condition specific PROMs |
| TJR | HOOS/KOOS or HOOS/KOOS – JR |
| Spine | ODI (low back), NDI (neck) |
| Shoulder | ASES |
General health related quality of life |
|
| VR-12 | |
| PROMIS 10 | |
Medical and MSK risk factors |
|
| back pain | |
| pain in other joints | |
Adverse Events |
|
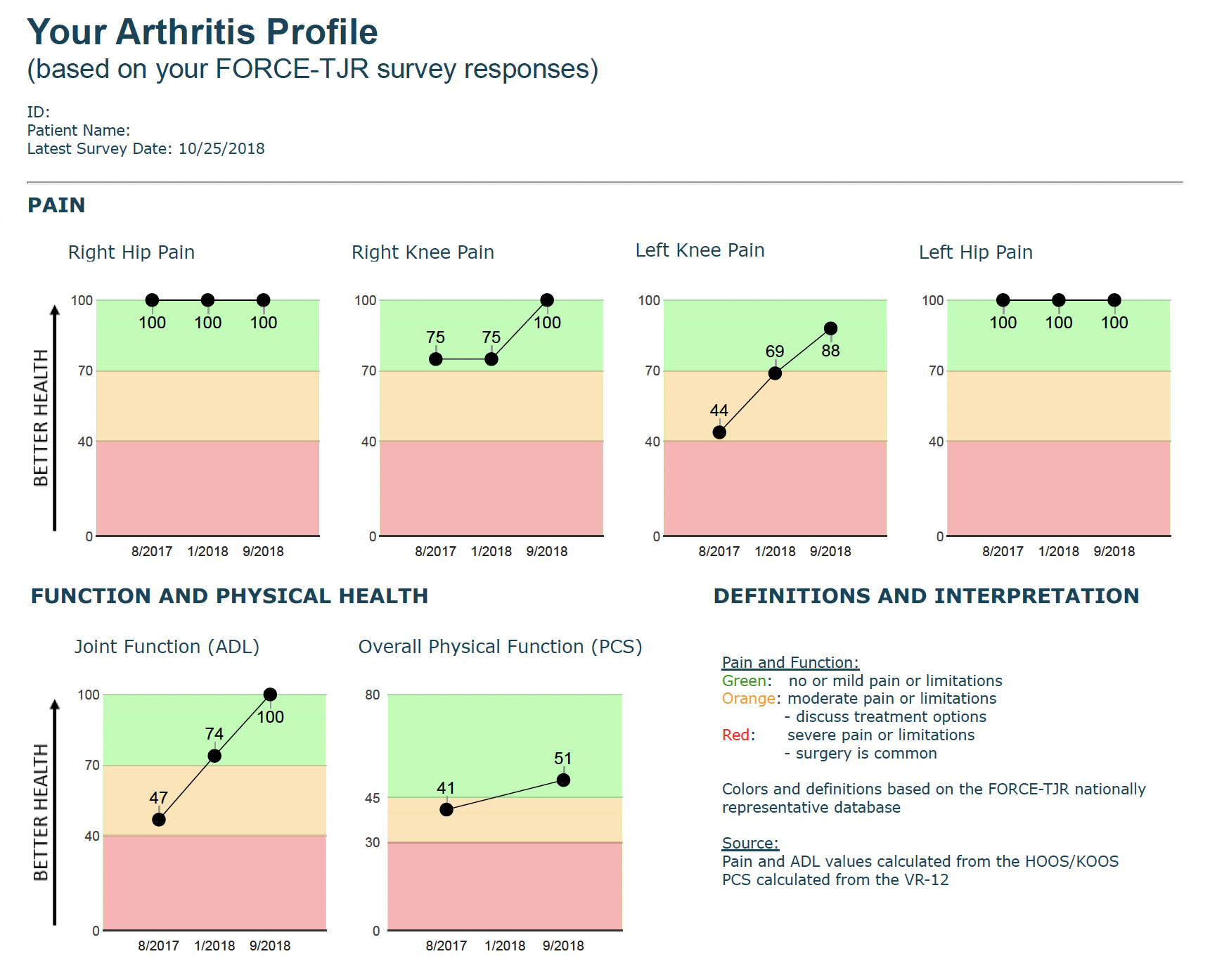
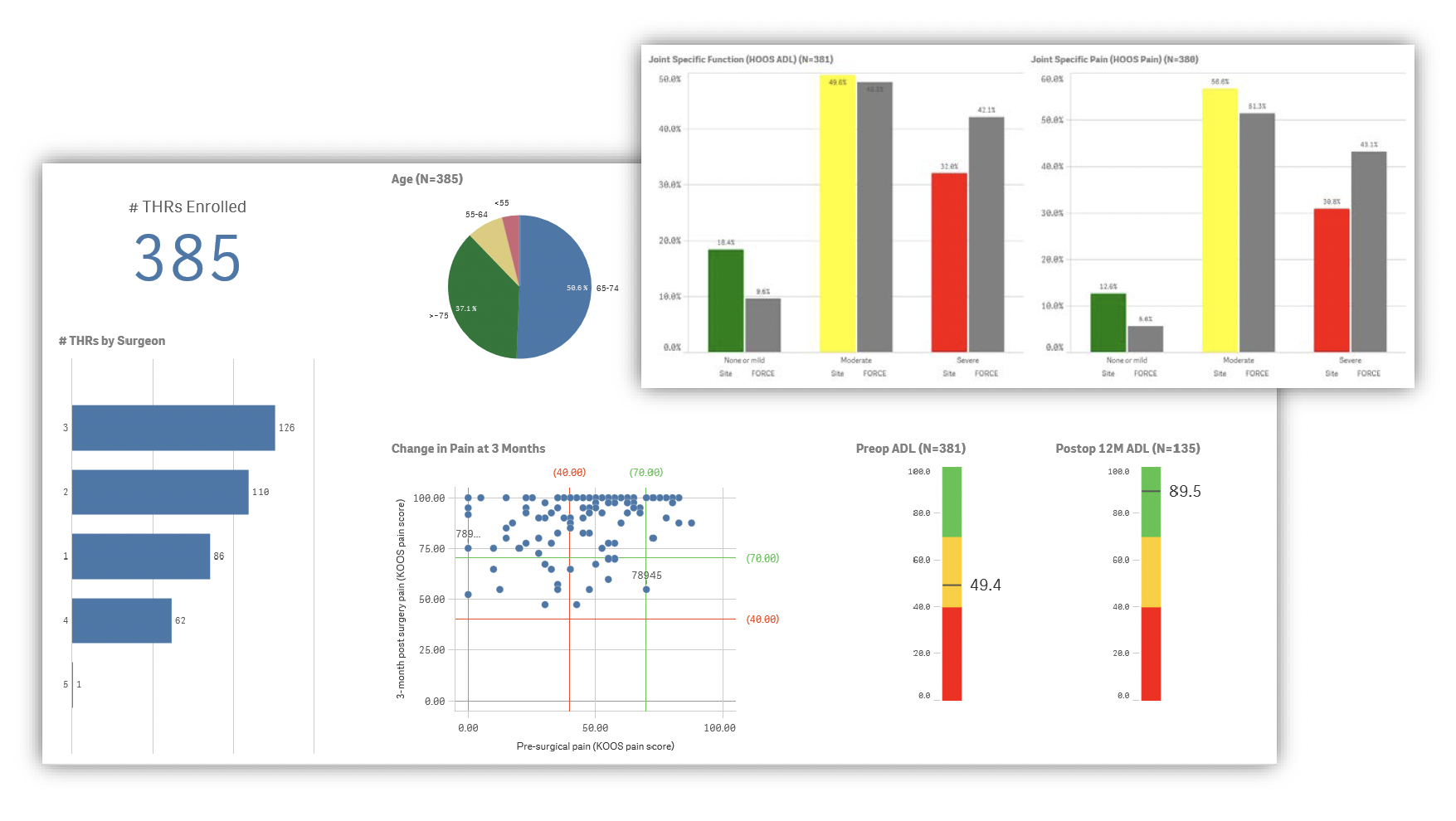
Our data are collected primarily from patients and include patient-reported outcome measures (PROM) of pain and function, early post-operative adverse events, and implant failures. In collaboration with patient and surgeon advisors, FORCE-TJR Research designed a real-time patient-reported pain and function monitoring report and tested it in surgeon offices. Scored and trended data, compared to national benchmarks, are available at the clinical visit to support treatment discussions between patients and surgeons.
In-office or at home direct-to-patient data collection to reduces burden on clinic staff Easy patient enrollment Automatically generated follow-up surveys with email reminders No IT interface required Collection of patient-reported outcome measures (PROMs), adverse events, and risk factors Measures satisfy CJR and MIPS quality reporting requirements.
The original FORCE-TJR cohort developed and demonstrated expertise in collecting and reporting TJR quality and patient-reported outcome measure (PROM) data. In contrast to others, FORCE-TJR collected post-TJR PRO data from over 85% of patients enrolled, exceeding the international goal of 70% completed.
FORCE-TJR was the first US TJR registry to identify and provide risk-adjusted national comparative reports for patient reported outcomes and 30-day readmissions.